Photosynthesis, Plant Respiration & Transpiration
Quantifying plant photosynthesis can be achieved by assessing CO2 or O2 gas exchange using the Qubit packages or customizable gas exchange measurement setups. The ready-to-use gas flux system Q-CO650 enables the monitoring of plant CO2 exchange and transpiration using a single flow-through channel. Analysing algal photosynthetic assimilation or bacterial respiration with the Q-FL23 involves measuring gas in the headspace of a cuvette. Photosynthesis packages available:
- Q-CO650 CO2 Plant Respiration Package (Leaves, Shoots, etc.)
- Q-FL23 CO2 Suspension Package (Algae, Fungi, Bacteria, etc.)
- Custom systems: CO2 Gas Analysers, O2 Analysers, Pumps and Flow Meters, etc.
Photosynthesis, Respiration and Transpiration Analysis - Q-CO650

Q-CO650 Plant CO2 Analysis Package, measures photosynthesis, respiration and transpiration in leaves when placed in a temperature controlled flow-through leaf chamber. The Plant CO2 Package can be used in the laboratory or in the field (with an optional battery pack). The Q-CO650 software automatically checks the reference levels of CO2 and water vapour and provides on-the-spot calculations of photosynthesis and transpiration rates. Applications include: Photosynthetic CO2 exchange rate, transpiration rate, water use efficiency studies, dark respiration, leaf, root and whole plant studies (roots and whole plants with optional ecosystem respiration chambers), and soil respiration or animal respirometry with additional components.
Photosynthesis Q-CO650 - Features
- Modular CO2 gas exchange measurement system - photosynthesis + H2O
- Temperature + light controlled flow-through chamber for leaves
- Automatic calculation of carbon exchange rates and transpiration
- Housed in a robust box for easy transport and set-up
- Laboratory and field use
- Full software suite - data capture to analysis
Optional:
- Arabidopsis and custom plant chambers available
- Battery pack, for field use
- Components interchangeable, add analysers and sensor as your research foci expand
| Components | Q-CO650 Package | OPEN | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | Photosynthesis Q-CO650 System | OPEN |
|
||
Operation Principle - Light and Temperature Control of the Q-CO650

- The Q-CO650 Plant CO2 Analysis Package uses a single infrared CO2 gas analyser (Q-S151). This analyser measures the concentration of CO2 gas entering and leaving a leaf chamber at different intervals. The user has control of the solenoid valve assembly, allowing alternation between reference and sample measurements. During light exposure, the package calculates the rate of photosynthetic CO2 fixation by analysing the difference between the influx and efflux CO2 levels (differential CO2) and taking into account the gas flow through the chamber. In the absence of light, CO2 exchange measurements provide valuable data on dark respiration. Continue reading
-
The Q-A113 LED Light delivers photosynthetically active radiation (PAR) to the leaf with minimal heat generation. This light source can deliver a maximum output of approximately 2000 µmol photons m-2 s-1 and is pre-calibrated in the software. This allows precise adjustment of light intensity without the need for a light meter. For leaf conductance calculations, the Q-S1730 Leaf Thermistor, located in the lower part of the leaf chamber, is essential. The Q-G1120 flow-through leaf chamber is equipped with temperature control. All analogue signals from the sensors are converted to digital signals via three built-in LabQuest mini interfaces, providing a total of nine channels. Data can be viewed, recorded and manipulated on a PC or MAC computer using Logger Pro software.
The Q-Box CO650 contains a humidity/ temperature sensor (Q-S161) that measures the relative humidity of the air before and after it passes through the leaf chamber. At the same time, the temperature at the rH sensor is also recorded. The difference in relative humidity between the incoming and outgoing gas, together with temperature, flow rate (through the leaf chamber) and leaf temperature, allows calculating transpiration rates. Each photosynthesis package includes customised experiment files to facilitate data collection, automated calculations and further analysis.
CO2 Exchange of Algae, Bacterial and Fungal Suspensions - Q-FL23 CO2 Analysis Package
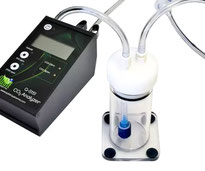
Q-FL23 Suspension CO2 Analysis Package, measures the CO2 exchange of algae, bacteria or fungi (or any kind of liquid suspensions incl. water samples) in the headspace of a cuvette. The CO2 gas released from the suspension into the headspace of the cuvette is dried and transferred to the Q-S151 CO2 analyser for measurement of CO2 levels (0-2000 ppm) in a flow-through system. Applications include algal photosynthesis, fermentation studies, biological activity of water samples, etc.
Q-FL23 Algae Photosynthesis - Features
- Cost-effective, modular system for measurement of CO2 released from any liquid culture
- Design significantly reduces the risk of liquid accidentally entering the gas analyser
- Includes all required components plus software and data interface
- Standard and custom-sized cuvettes / measurement cells available
| Components | Q-FL23 Package | OPEN | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| References | Algae Q-FL23 CO2 Exchange Package | OPEN |
|
||
Operation Principle of the Q-FL23 Algae CO2 System
- Gas enters the cuvette (standard 80 ml, or custom made) via the inlet port and an air stone located below the liquid level. The cuvette is filled with the sample of choice, e.g. algae, bacterial or fungal suspensions. The entering gas causes bubbling and mixing of the sample. CO2 from the (algal) suspension is released into the headspace above the liquid and exits the chamber via the gas outlet. Continue reading
-
The gas leaving the chamber passes through a PTFE filter and a Nafion rH equilibrator before entering the drying column and the Q-S151 CO2 analyser. Should any liquid accidentally escape from the algal chamber, the PTFE filter will prevent it from entering the Q-S151 CO2 analyser and the Nafion rH equilibrator will reduce the water vapour in the gas to ambient levels. Illumination of the algal culture is provided by the A113 LED light source, which is software calibrated for PAR output in µmol photon m-2 s-1; other light sources suitable for the experimental set-up may be used.
Fundamentals of gas exchange measurements
Most modern gas exchange systems operate as 'open' systems, where a continuous stream of gas is passed over a leaf placed in a well-mixed measuring chamber. Infrared gas analysers (IRGA) are used to measure gas properties, primarily the concentrations of CO2 and H2O. Measurements are taken both before the gas enters the leaf chamber (reference IRGA) and after it leaves the chamber (sample IRGA). By knowing the gas flow rate entering the system (μ0; μmol air s-1) and the leaf area enclosed in the chamber (s; m2), the CO2 assimilation rate (A; μmol CO2 m-2 s-1) and the transpiration rate (E; mmol H2O m-2 s-1) at the leaf level can then be calculated from the differences in CO2 and H2O concentrations between the sample and reference IRGAs. Often one IRGA is used, and the gas flow is cost effectively switched between the reference and measurement channels.
The recent review by Busch et al. (2024) elucidates the essential principles underlying photosynthetic gas exchange and provides guidance on best practices for obtaining and accurately interpreting measurements. This review provides a solid foundation in gas exchange techniques for the newcomer and serves as a valuable reference point for the advanced researcher. Read more on how to get gas exchange measurements right and make them fit your research questions:
Review: Busch FA, Ainsworth EA, Amtmann A, Cavanagh AP, Driever SM, Ferguson JN, et al. (2024) A guide to photosynthetic gas exchange measurements: Fundamental principles, best practice and potential pitfalls. Plant, Cell & Environment. https://doi.org/10.1111/pce.14815
Classic reading: Von Caemmerer S, Farquhar GD. (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 153:376-87.
To determine photosynthetic parameters via Chlorophyll Fluorescence Imaging, consider the reliable BLT-PlantView 230F Imager.
If Soil Respiration is of interest, consider the Soil Package Q-SR1LP - offering a complete, ready to go solution for the lab and field.
For a wide range of individual components, see e.g. Gas Analyzers, Flow Monitors & Pumps, Pressure Sensors, and Gas Control Systems.
For classroom/hands-on experimental kits for photosynthesis and plant respiration, see the Q-Teach Plant Package.